Connect your integrations
Manage everything via one platform. Your team, your clients, your leads, everything – Using just the Dashboard.
![]() Head Of Regulatory Affairs UK
Head Of Regulatory Affairs UK ![]() AstraZeneca
AstraZeneca
 JOB TITLE
JOB TITLEHead Of Regulatory Affairs UK
 COMPANY NAME
COMPANY NAMEAstraZeneca
 LOCATION
LOCATIONStevenage, United Kingdom
 EXPERIENCES
EXPERIENCESHead Of Regulatory Affairs UK
 Cambridge, United Kingdom
Cambridge, United Kingdom
July 2021 - Present
AstraZeneca is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialisation of prescription medicines. Our Purpose is to push the boundaries of science to deliver life-changing medicines. We believe the best way we can achieve our Purpose is to put science at the centre of everything we do. Science defines who we are. It is why we come to work every day and is part of our DNA. But this is only half of the story. We know that we do not have all the answers. We want to share ideas because we believe it results in better medicines. We want the way we work to be inclusive, open and collaborative. This approach runs through all that we do. We focus on three main therapy areas – Oncology, Cardiovascular & Metabolic Disease (CVMD) and Respiratory – and we are also selectively active in the areas of autoimmunity, neuroscience and infection. To put ourselves in the best position to push the boundaries of science, we seek to leverage our combination of capabilities, which encompass both small molecules and biologics, and include immunotherapies and developing innovative delivery devices that can offer choice to patients. These are reinforced by a strong focus on personalised healthcare capabilities, which aim to match medicines only to those patients who will benefit from them. Our teams also work alongside the world's leading academic and biotech research institutions to stimulate innovation and evaluate emerging technologies such as Modified RNA and CRISPR genome editing. AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. For more information, visit www.astrazeneca.com. Community Guidelines: bit.ly/2MgAcio
Regulatory Affairs Lead
October 2015 - July 2021
Regulatory Affairs Manager
June 2013 - October 2015
Regulatory Executive
 Luton, United Kingdom
Luton, United Kingdom
October 2011 - May 2013
Quality Assurance And Regulatory Affairs Manager
October 2003 - October 2011
ALT
January 2000 - January 2002

1 EMAIL(S)
k***@astrazeneca.com

3 PHONE NUMBER(S)
+44 0***
+44 2***
+44 0***
 Director, Statistical Programming
Director, Statistical Programming
 AstraZeneca
AstraZeneca
 Cambridge, United Kingdom
Cambridge, United Kingdom
s***@astrazeneca.com
+44 *** +3
 US & Global Insights & Analytics. Vaccines And Immune Therapies. Senior Director
US & Global Insights & Analytics. Vaccines And Immune Therapies. Senior Director
 AstraZeneca
AstraZeneca
 Cambridge, United Kingdom
Cambridge, United Kingdom
e***@astrazeneca.com
+60 *** +4
 Director Of Business Planning And Operations
Director Of Business Planning And Operations
 AstraZeneca
AstraZeneca
 Cambridge, United Kingdom
Cambridge, United Kingdom
m***@astrazeneca.com
+44 *** +3
 Diagnostic Manager
Diagnostic Manager
 AstraZeneca
AstraZeneca
 Haywards Heath, United Kingdom
Haywards Heath, United Kingdom
s***@astrazeneca.com
+44 *** +3
 Security Manager
Security Manager
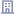 AstraZeneca
AstraZeneca
 Great Abington, United Kingdom
Great Abington, United Kingdom
b***@astrazeneca.com
+44 *** +3
Kaspr will display your prospect’s contact information the moment you visit their LinkedIn profile. Quick! Isn’t it?
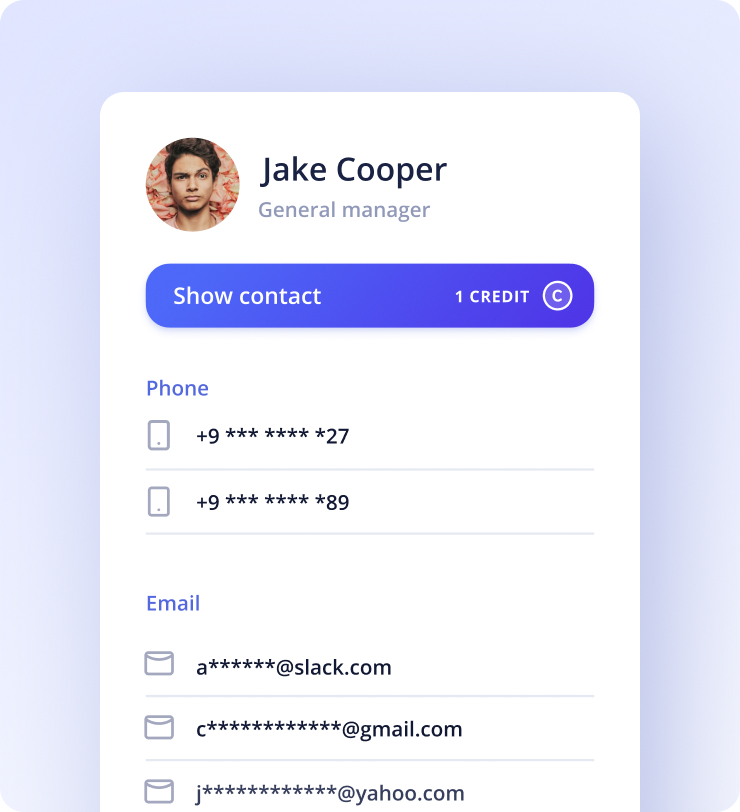
Kaspr lets you send automated follow up messages to your leads. Send them on your chosen day and time.
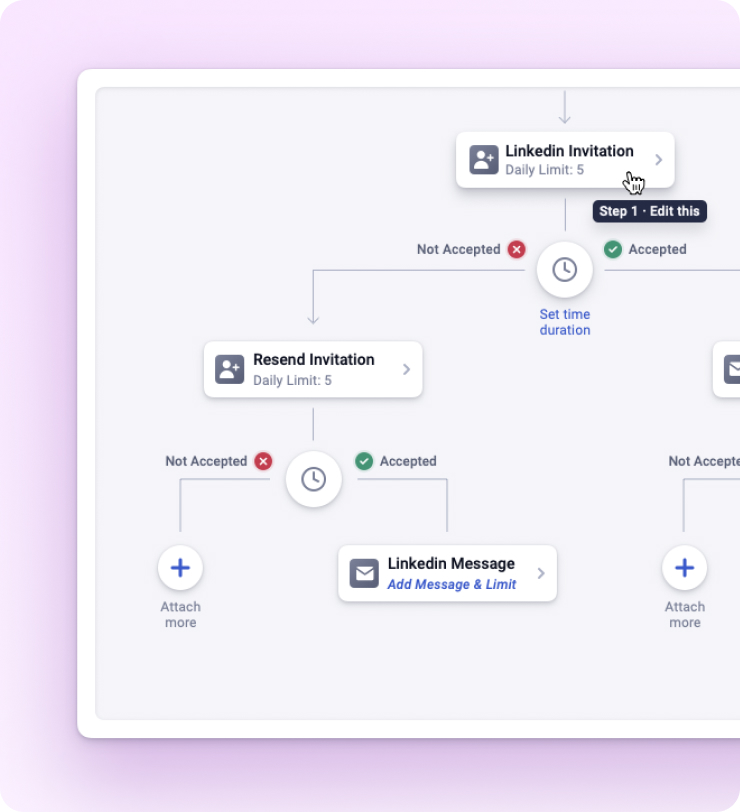
Manage everything via one platform. Your team, your clients, your leads, everything – Using just the Dashboard.