Connect your integrations
Manage everything via one platform. Your team, your clients, your leads, everything – Using just the Dashboard.
Aseptic Manufacturing Specialists - Leading the Way in Viral Vectors
 DESCRIPTION
DESCRIPTIONSymbiosis Pharmaceutical Services is a contract manufacturing organisation (CMO) that specialises in the GMP manufacture and sterile fill/finish of vials for clinical trials and low-volume commercial supply. Regulatory compliance, technical capability and operational flexibility are at its core. Manufacturing takes place in a purpose-built MHRA-licensed and FDA approved facility, enabling the CMO to handle products that require aseptic liquid filling and lyophilisation for a range of complex biologics, viral vectors for use in gene therapies and small molecule drugs. Offering fast access to manufacturing slots and accelerated release of drug product, Symbiosis is primed to meet demand for small-scale, fast-turnaround drug product manufacturing, while maintaining regulatory compliance to the highest standards. Symbiosis specialises in the sterile fill/finish of biologic and small molecule products into vials, which are either liquid or lyophilised formulations. Capabilities include the ability to handle and fill antibodies, proteins, peptides, nucleic acids, cytotoxic, cytostatic and highly potent APIs including ADCs along with conventional small molecules. Symbiosis saves our clients invaluable development time. With short lead times to manufacture and faster release of sterile drug product, we get you into phase I/II quickly. Our focus is service excellence through flexibility, responsiveness and timeliness. If you need a drug filled aseptically, come and speak with Symbiosis...we will exceed your expectations.
 ADDRESS HQ
ADDRESS HQStirling, United Kingdom
 COMPANY NAME
COMPANY NAMESymbiosis Pharmaceutical Services
 CREATION DATE
CREATION DATE2011
 INDUSTRY
INDUSTRYBiotechnology
 SPECIALITIES
SPECIALITIESSmall-scale GMP manufacture of sterile drug product, Lyophilization, Cytotoxics/Highly potent drugs, Biologics, Liquid, Vial filling, Sterile Fill Finish
 LINKEDIN COMPANY PAGE
LINKEDIN COMPANY PAGEhttps://linkedin.com/company/symbiosis-pharmaceutical-services-limited
 Patterns
Patterns Example
Example Score
Score{first}.{last}@symbiosis-pharma.com
john.doe@symbiosis-pharma.com
91%
| COMPANY | INDUSTRY | LOCATION | NO OF EMPLOYEES | ||
| FLUOPTICS | Biotechnology | Grenoble, France | 10-50 | See company details | See employee details |
| Eurofins | Biotechnology | Luxembourg, Luxembourg | 10000 + | See company details | See employee details |
| PsiOxus Therapeutics Ltd | Biotechnology | Abingdon, United Kingdom | 50-200 | See company details | See employee details |
| AB-BIOTICS | Biotechnology | San Cugat Del Vallés, Spain | 10-50 | See company details | See employee details |
| BioMérieux | Biotechnology | Marcy L'etoile, France | 10000 + | See company details | See employee details |
Symbiosis Pharmaceutical Services has 113 employees.
Symbiosis Pharmaceutical Services was founded in 2011.
Symbiosis Pharmaceutical Services's LinkedIn page currently has 3337 followers.
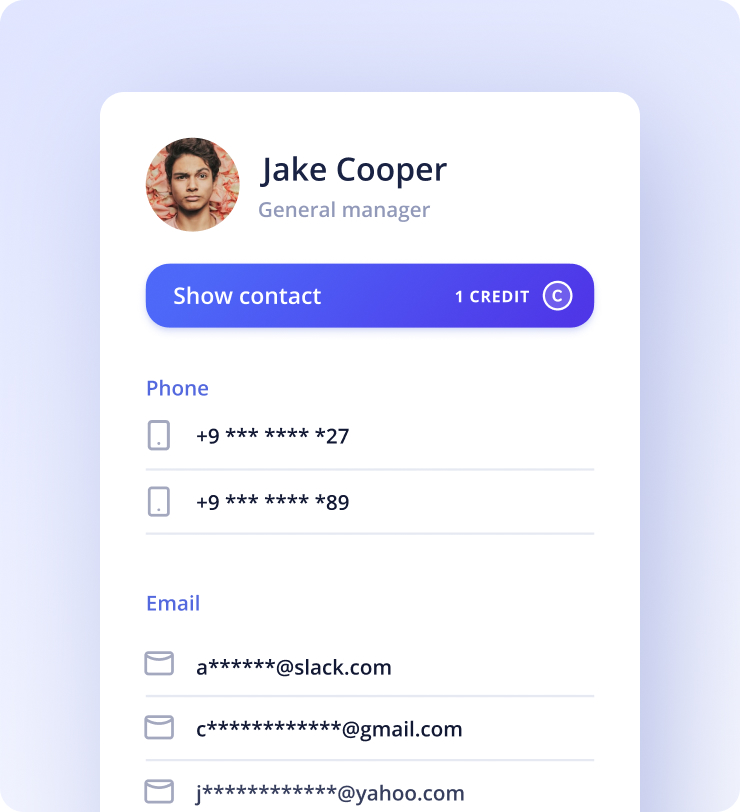
Kaspr will display your prospect’s contact information the moment you visit their LinkedIn profile. Quick! Isn’t it?
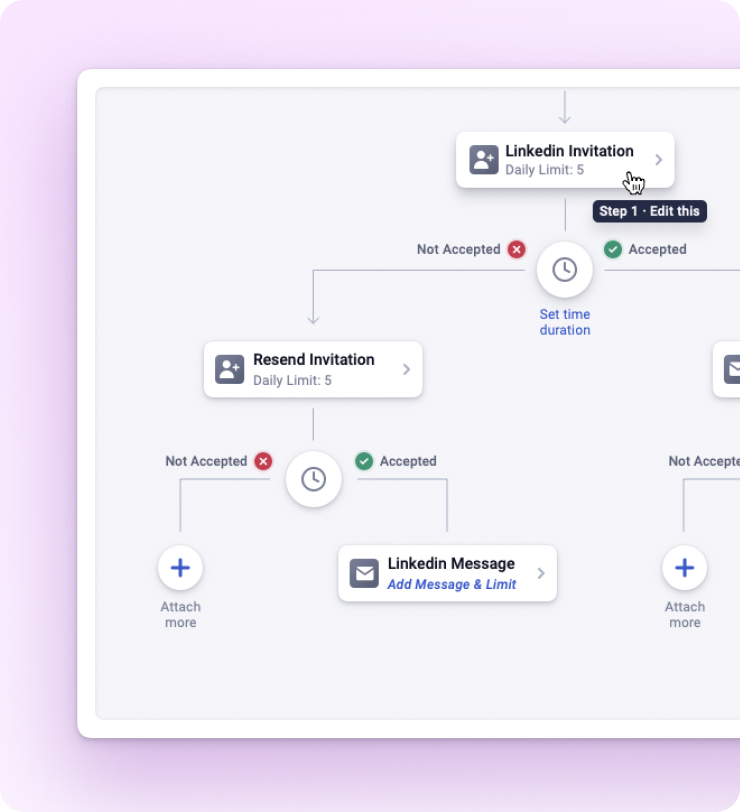
Kaspr lets you send automated follow up messages to your leads. Send them on your chosen day and time.
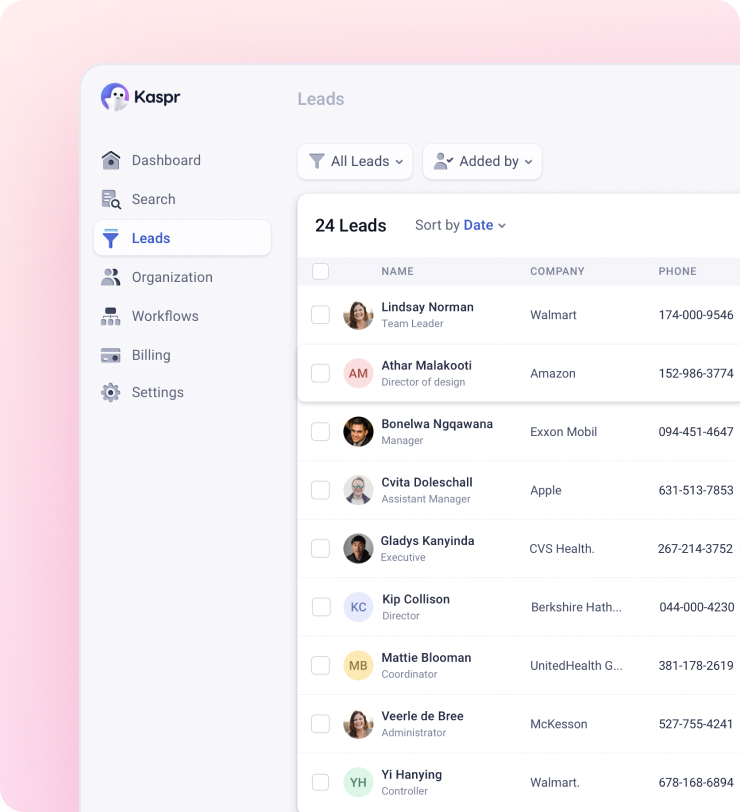
Manage everything via one platform. Your team, your clients, your leads, everything – Using just the Dashboard.